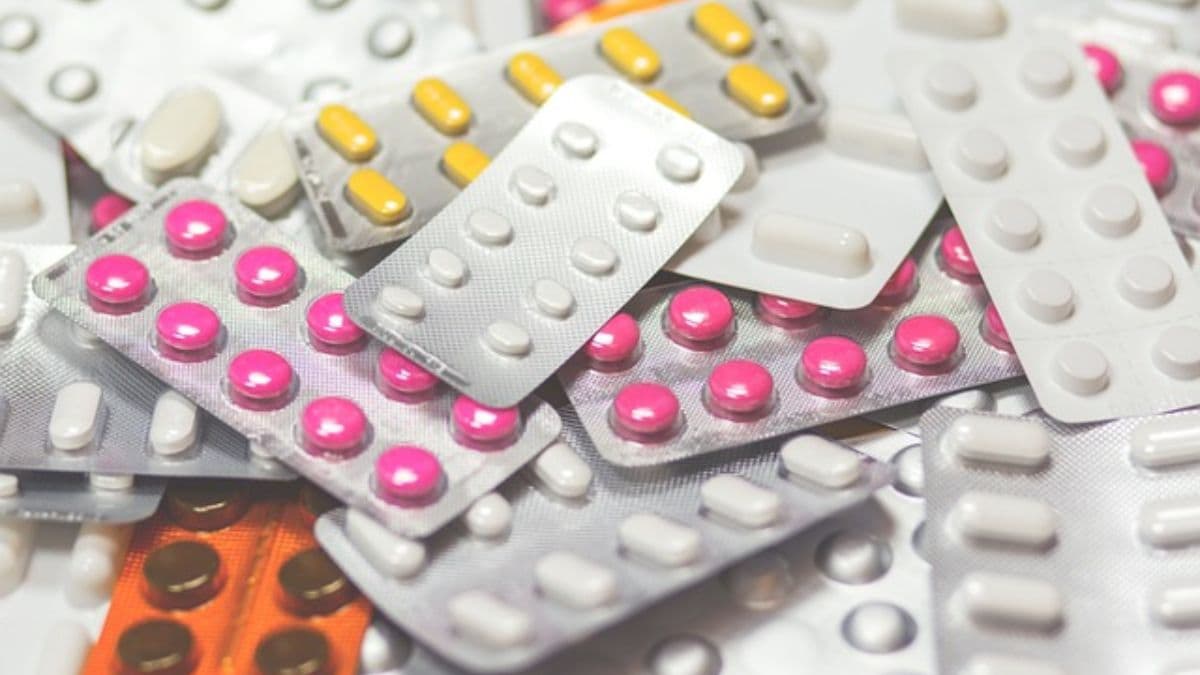
In a shocking revelation, India’s top drug regulator, the Central Drugs Standard Control Organisation (CDSCO), has flagged over 50 commonly used medications, including household names like Paracetamol and antacid Pan D, for failing to meet safety and quality standards. These are not obscure drugs, but essentials found in millions of homes across the country. From vitamins and calcium D3 supplements to antiacid Pan-D and diabetes and blood pressure medications have been listed as “not of standard quality” by the CDSCO.
These findings, released in a recent alert, have sparked significant worry among consumers and healthcare professionals alike. Here’s what we know about the situation Which medicines have been flagged? Several well-known medications have been flagged by CDSCO, following random monthly testing by state drug officers. CDSCO is the sole authority that grants no-objection certificates to pharmaceutical companies that manufacture drugs that are unapproved, new, or banned for the sole purpose of exports in India.
The alert issued in August revealed over 50 popular drugs failing to meet quality standards, spanning pain relievers, antibiotics, and supplements from major pharmaceutical manufacturers. Among the flagged medications are Paracetamol 500 mg tablets, the anti-diabetic drug Glimepiride, and high blood pressure medication Telma H (Telmisartan 40 mg). Acid reflux treatment Pan D and calcium supplements Shelcal C and D3 were also on the list.
Additionally,.