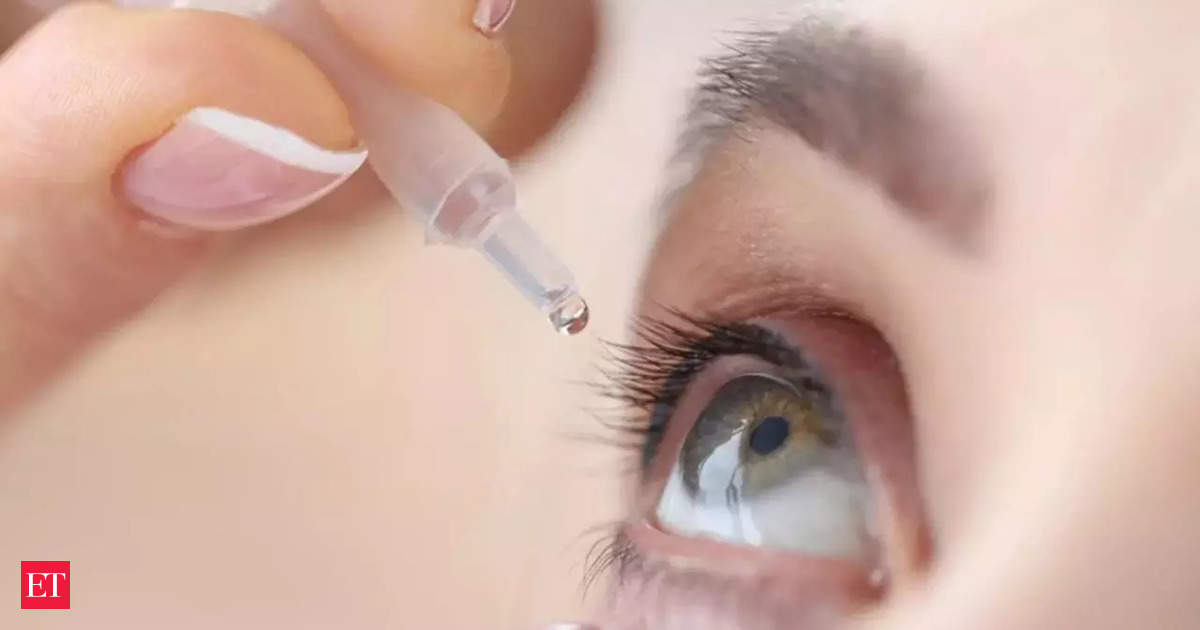
Mumbai-based Entod Pharmaceuticals on Thursday said its eye drop PresVu does not intend to replace reading glasses or non-invasive options for presbyopia , or lack of clarity in near vision, days after India's drug regulator asked the company to stop the manufacture and sale of its new eye drop. The regulatory action to suspend the manufacture and sale of the eye drop came after the licensing authorities said the company did not get its clearance on its claim that the medicine reduces dependency on reading glasses for those affected by presbyopia. The company's CEO, Nikkhil K Masurkar, Thursday said the eye drop "is a therapeutic option.
" Masrukar met the drug controller earlier this week and said that it was justified on the part of DCGI to suspend the approval. He had met the DCGI to address concerns surrounding Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% w/v (PresVu eye drops ), which were approved for treating presbyopia in adults on August 6.
However, the drug controller suspended its permission on September 10 following potentially misleading claims. (You can now subscribe to our Economic Times WhatsApp channel ).