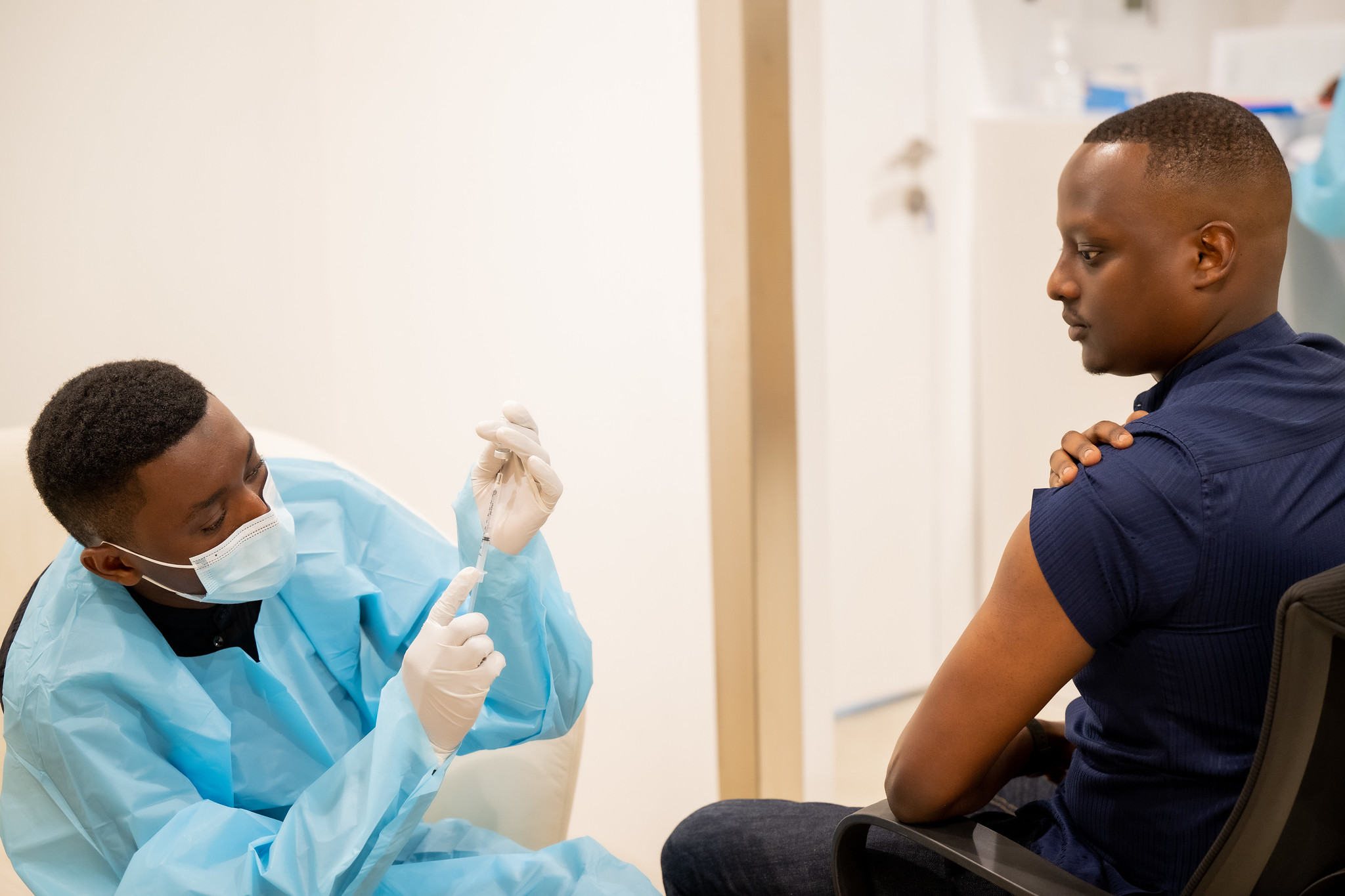
Photo caption: Rwanda Minister of State for Health, Yvan Butera, was among those who received Sabin’s investigational vaccine in the open-label clinical trial. WASHINGTON, Oct. 31, 2024 (GLOBE NEWSWIRE) -- In continued collaboration with Rwanda to address the Marburg virus outbreak, the Sabin Vaccine Institute has dispatched approximately 1,000 additional investigational vaccine doses for a randomized clinical trial arm within the ongoing open-label study.
More than 1,500 frontline workers have already been vaccinated in Rwanda with the Sabin vaccine. Under the updated protocol, sponsored by the Rwanda Biomedical Center, approximately 1,000 at-risk individuals, including mine workers, will receive Sabin’s single-dose investigational vaccine in a 1-to-1 randomization. Half will receive the vaccine immediately, and the other half 21 days later to align with the end of the disease incubation period.
Genomic sequencing of the index case (first identified case in an infectious disease outbreak) in Rwanda suggests a zoonotic origin, with strains similar to those found in fruit bats in a mine. The new trial arm will assess safety, immunogenicity, and efficacy. Pending a request from Rwandan officials and authorization from the U.
S. Administration for Strategic Response and Preparedness, Sabin plans to supply additional vaccines for this portion of the trial. “As Rwandan health officials determined, the most expeditious and effective way to reach this new group of people impact.