Company is now funded for and focused on New Drug Applications (NDAs) for NRX-100 (ketamine) and NRX-101 Audit of HOPE Therapeutics is now complete, SEC filing of spinout this quarter Key Milestones Secured $10.8 - $16.3 million in convertible-debt funding from an institutional investor; funds targeted to support FDA New Drug Applications for NRX-100 (ketamine) and NRX-101.
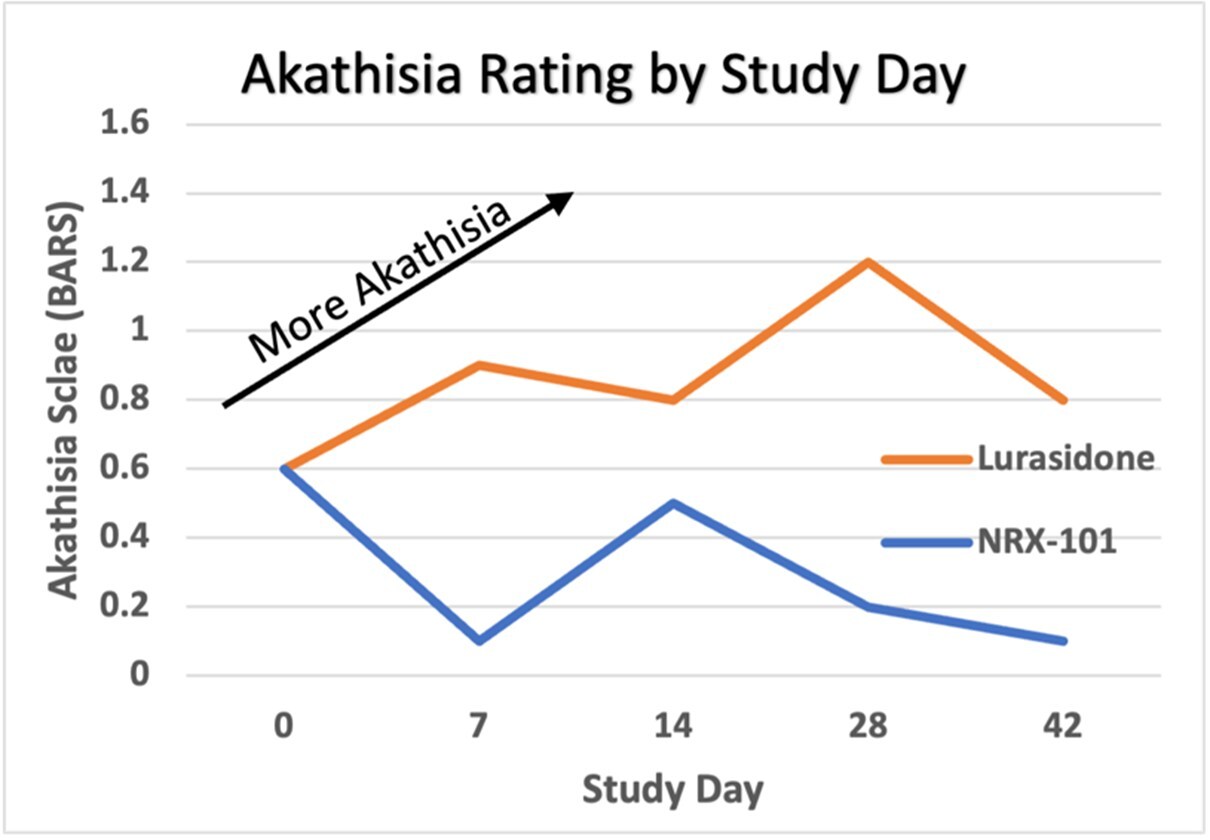
Replacement funding entails substantial reduction in interest rate, conversion discount, and other financial terms compared to prior debt Retirement of Streeterville debt and settlement of litigation at a substantial discount to litigation claims NRX-100 NDA for suicidal depression based on data from four clinical trials in nearly 1000 participants demonstrating highly significant efficacy compared to placebo, active comparator, and electroshock therapy Ketamine findings have just been confirmed in published 43,000 person cohort study 1 Phase 2b /3 trial of NRX-101 in suicidal patients with bipolar depression demonstrated depression efficacy comparable to standard of care and significant reduction of akathisia (P=0.025) and time to sustained remission from suicidality (P=.05).

Presented at the annual meeting of the American Society of Clinical Psychopharmacology. Profile demonstrates possible best in class bipolar depression medication Company plans to file a New Drug Application (NDA) for Accelerated Approval under Breakthrough Therapy Designation and Priority Review of NRX-101 in treatment of bipolar depression in people akathisia or suicidality, based on the Phase 2b /3 and STABIL-B data Stability data continues to mature on the three manufacturing lots required for the NRX-100 (IV ketamine) NDA filing and the Company announced alignment with FDA on its Pediatric Study Plan for NRX-100, also a requirement for filing an NDA HOPE Therapeutics, the Company's wholly owned subsidiary, is focused on developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression and related disorders. HOPE is planned to be spun out as a separate company to be owned by NRx, current NRx shareholders, and new investors.
This effort will be funded apart from NRx. Appointed Dr. Dennis McBride , a Neuroscience, Information Technology and Medical Technology Veteran, to its Board of Directors Management to host a conference call August 14, 2024 , at 4:30 PM ET RADNOR, Pa.
, Aug. 14, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP ) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced its financial results for the quarter and year to date ended June 30, 2024 , and provided a business update.
.