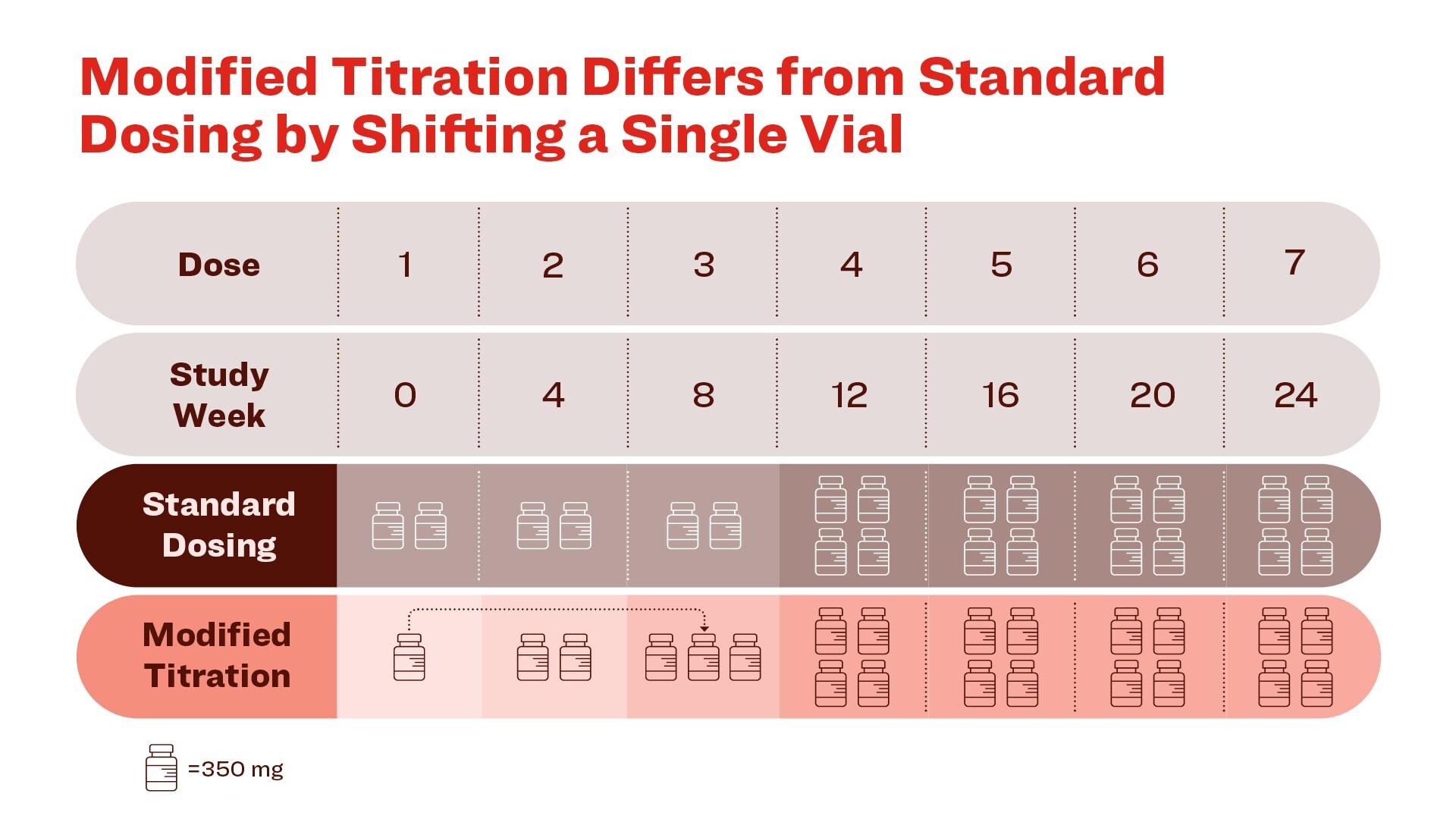
A change in the initiation of donanemab dosing, shifting one vial of donanemab from the first infusion to the third infusion, lowered ARIA-E to 14% compared to 24% in patients receiving the standard dosing regimen used in the pivotal Phase 3 clinical trial Reduction of amyloid plaque and P-tau217 on this modified titration was comparable to patients receiving the standard dosing regimen Lilly intends to submit this data to global regulators for a potential label update for Kisunla (donanemab-azbt) INDIANAPOLIS , Oct. 29, 2024 /PRNewswire/ -- Eli Lilly and Company (NYSE: LLY ) today announced positive results from the TRAILBLAZER-ALZ 6 Phase 3b study, showing a reduction in amyloid-related imaging abnormalities with edema/effusion (ARIA-E) at the 24-week primary endpoint for people receiving a slightly modified titration of donanemab in adults with early symptomatic Alzheimer's disease (AD). 1 Donanemab is approved under the brand name Kisunla TM in the United States , Japan , Great Britain and other countries.
These data were presented at the 17th Clinical Trials on Alzheimer's Disease (CTAD) Conference in Madrid, Spain . TRAILBLAZER-ALZ 6 is a multicenter, randomized, double-blind, Phase 3b study to investigate the impact of different dosing regimens of donanemab on the rates of ARIA-E and amyloid clearance in adults with early symptomatic AD, which includes mild cognitive impairment (MCI) and the mild dementia stage of disease. "Lilly is committed to continuing research to .