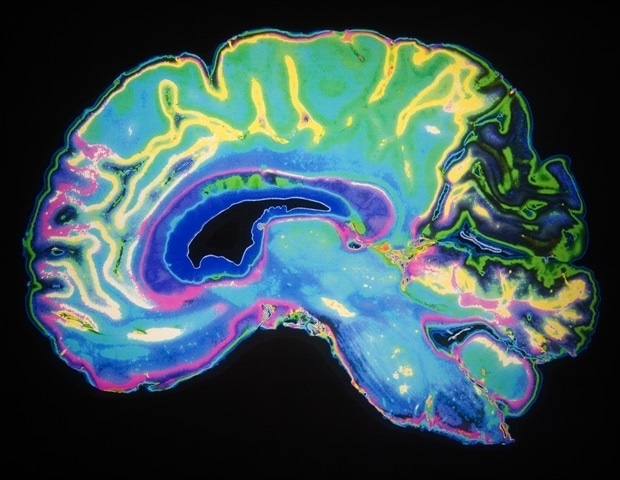
A radiopharmaceutical therapy that has successfully extended progression-free survival for patients with neuroendocrine tumors shows early promise for delivering similar benefits to patients with difficult-to-treat meningioma, a type of brain tumor. Findings of the nonrandomized phase II study will be presented today at the American Society for Radiation Oncology (ASTRO) Annual Meeting. "We've found a therapy with a meaningful signal for effectiveness and safety for people with refractory meningioma, a condition with no standard treatment options," said Kenneth W.
Merrell, MD, principal investigator (PI) of the trial and a radiation oncologist at the Mayo Clinic Alix School of Medicine in Rochester, Minn. "Nearly 80% of patients in our study were progression-free after six months. This rate greatly surpassed the benchmark from prior research, suggesting that radiopharmaceuticals are a promising therapeutic agent for these patients.
" Meningiomas are tumors that grow in the connective tissue surrounding the brain and spinal cord. They are the most common type of primary brain tumor, and while they typically do not spread to other parts of the body, they can grow uncontrollably and lead to disabling and deadly compression of the nerves and brain. Standard treatment for patients with meningiomas is either surgical removal or external beam radiation therapy when tumors grow in areas where surgery is too dangerous, such as close to the brain stem or spinal cord.
But for the portion.