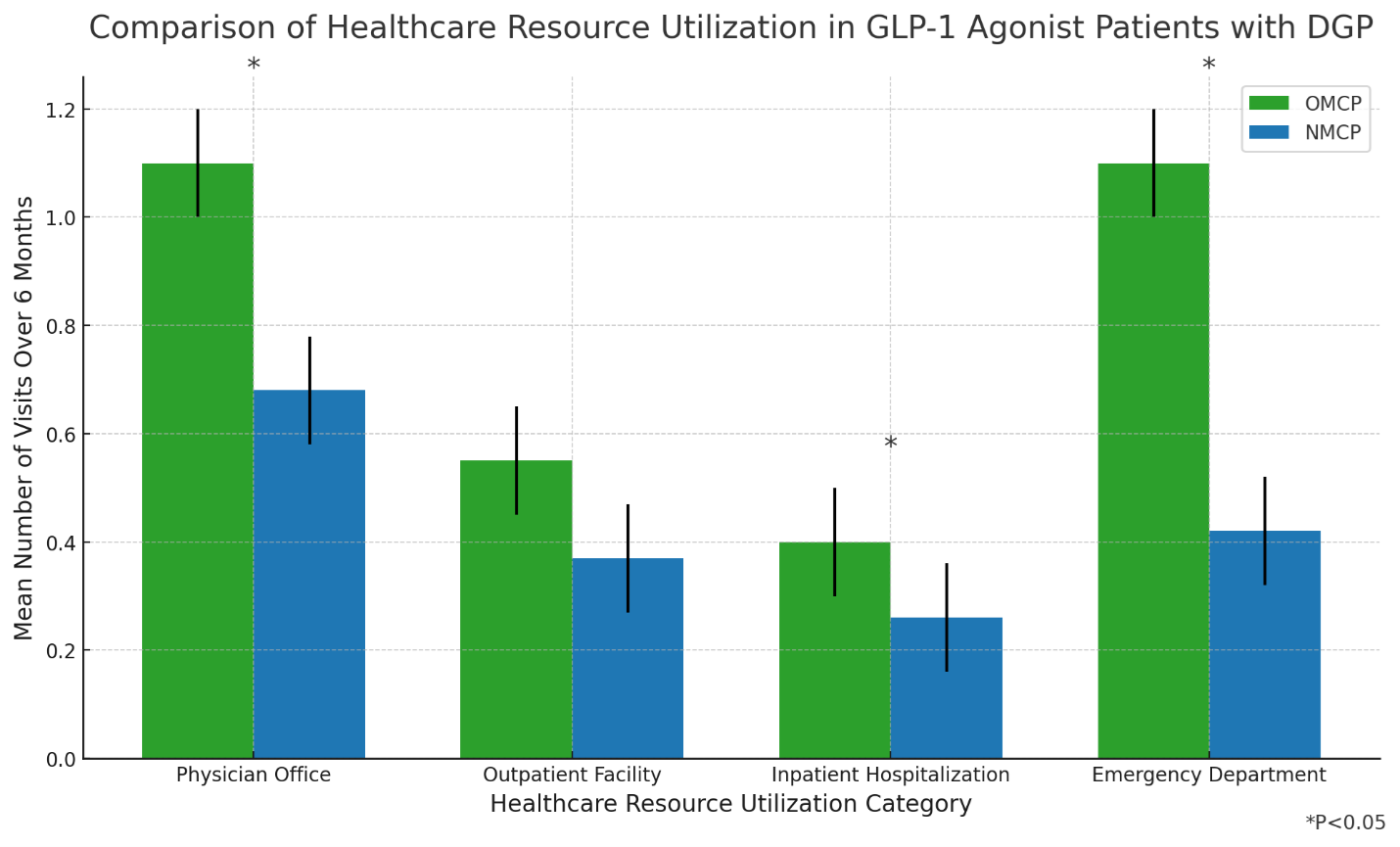
Analysis of real-world data compared patients on GIMOTI (n=51) to Oral Metoclopramide (n=41), both taking GLP-1s, showing significant statistical improvement for GIMOTI over Oral Metoclopramide in All Cause Emergency Department Visits (-91%, p=0.001), All Cause Office Visits (-41%, p=0.027) and All Cause Hospital Outpatient Visits (-89%, p=0.
032) within 6-month index period Data presented at American College of Gastroenterology (ACG) 2024; the submission garnered both the Presidential Poster Award as one of the top 5% of data accepted to the conference and selected as the Outstanding Research Award in the Stomach Category First study to show Gimoti’s potential as supportive care for GLP-1 therapy SOLANA BEACH, Calif., Oct. 28, 2024 (GLOBE NEWSWIRE) -- Evoke Pharma, Inc.
(NASDAQ: EVOK), a specialty pharmaceutical company focused on developing treatments for gastrointestinal (GI) diseases, with a particular emphasis on GIMOTI ® (metoclopramide) nasal spray, together with EVERSANA, a leading provider of global commercial services to the life sciences industry, today announced the presentation of data for GLP-1 users with diabetic gastroparesis using GIMOTI at the American College of Gastroenterology (ACG) 2024 Annual Meeting. The real-world retrospective study evaluated the impact of GIMOTI (metoclopramide nasal spray) in patients with diabetic gastroparesis (DGP) who were concurrently using GLP-1 receptor agonists. GLP-1 drugs are commonly prescribed for type 2 diabetes, and.