SOUTH SAN FRANCISCO, Calif., Sept. 09, 2024 (GLOBE NEWSWIRE) -- Cytokinetics, Incorporated (Nasdaq: CYTK) today announced that data from the Phase 1 study of CK-4021586 (CK-586) were presented in a poster session at the American College of Clinical Pharmacology (ACCP) Annual Meeting in Bethesda, MD.
The study met its primary and secondary objectives to assess the safety, tolerability and pharmacokinetics (PK) of single and multiple oral doses of CK-586. The data support the advancement of CK-586 to a Phase 2 clinical trial in patients with heart failure with preserved ejection fraction (HFpEF) which is expected to begin in Q4 2024. CK-586 is a cardiac myosin inhibitor in development for the potential treatment of a subgroup of patients with symptomatic HFpEF with hypercontractility and ventricular hypertrophy.

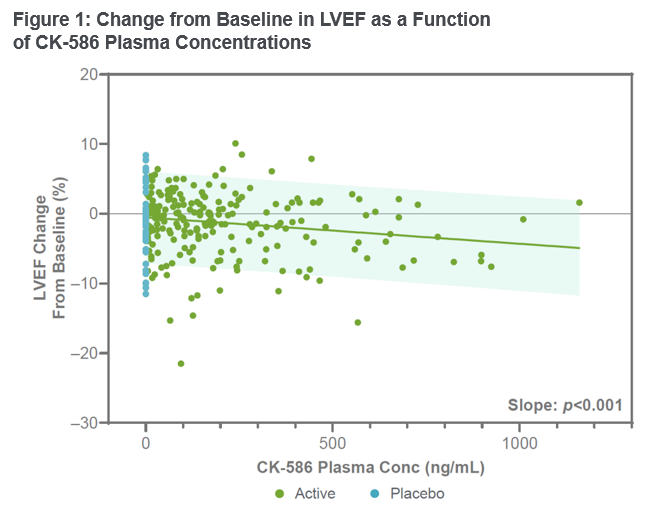
“The results from this Phase 1 study replicate pre-clinical findings that show CK-586 directly reduces cardiac contractility at the level of the sarcomere. Importantly, CK-586 was observed to have a shallow and predictable PK/PD relationship and half-life that enables a once-daily fixed dosing regimen in patients with HFpEF,” said Stuart Kupfer, M.D.
, Senior Vice President, Chief Medical Officer. “Preparations are underway for a Phase 2 clinical trial of CK-586 in a subset of patients with HFpEF that we plan to start in the fourth quarter.” The primary objective of this Phase 1 double-blind randomized, placebo-controlled, single and multiple ascending dose clini.