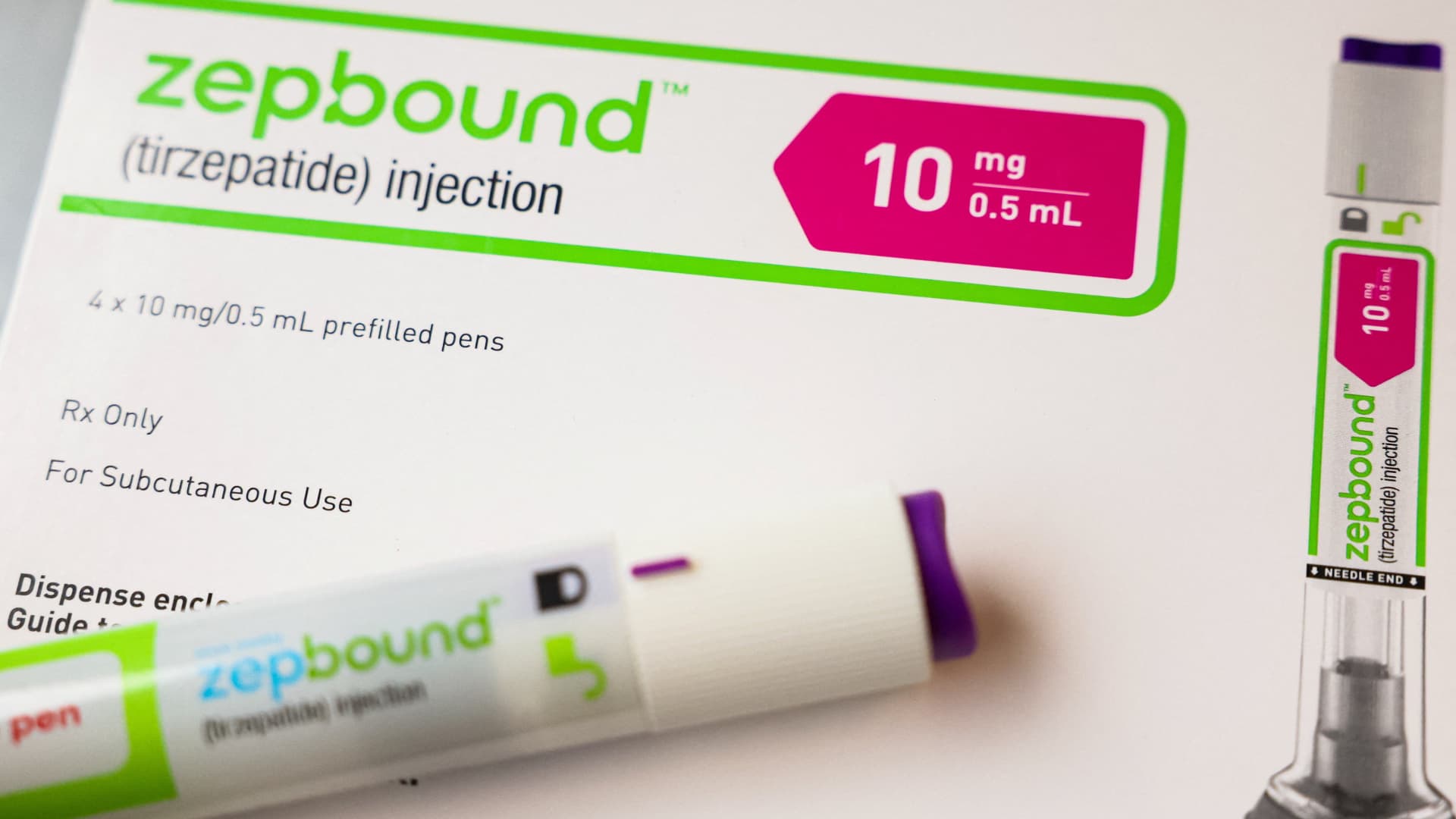
In this article LLY Follow your favorite stocks CREATE FREE ACCOUNT An injection pen of Zepbound, Eli Lilly’s weight loss drug, is displayed in New York City, U.S., December 11, 2023.
Brendan McDermid | Reuters Eli Lilly on Wednesday said its highly popular weight loss drug Zepbound showed the potential to treat patients with the most common sleep-related breathing disorder in two late-stage clinical trials. The initial results add to the long list of potential health benefits of weight loss and diabetes treatments, which have skyrocketed in demand over the last year despite their high prices and spotty insurance coverage. Zepbound was more effective than a placebo at reducing the severity of obstructive sleep apnea , or OSA, in patients with obesity after a year, according to preliminary data from both trials.
The pharmaceutical giant said it plans to present the results at an upcoming medical conference and submit it to the U.S. Food and Drug Administration and regulators in other countries in the middle of the year.
Eli Lilly previously announced that the FDA granted Zepbound "fast track designation" for patients with moderate-to-severe OSA and obesity. That designation expedites the review of drugs that treat a serious or life-threatening condition and fill an unmet medical need. The results are an early sign of hope for the estimated 80 million patients in the U.
S. suffering from OSA, which refers to interrupted breathing during sleep due to narrowed or blocked airways.